Pocket Pets and Exotics
Member Only Content
Members Links
Printer Articles
- Anatomy
- Dental Extractions of Single Rooted Teeth
- Direct Pulp Capping (Partial Coronal Pulpectomy)
- Extractions
- Feline Dental Radiography
- Homecare
- Infra-orbital nerve block in the dog.
- Introduction to Anaesthesia
- Members Lectures
- Paedodontics
- Periodontal Disease 3 – Saving the Periodontally Diseased Tooth
- Periodontology
- Pocket Pets and Exotics
- Procedure Videos
- Reference Manuals
- Rest acid etch
- Restoratives – Composite Bonding
- Setting Up a Dental Surgery – Dental Equipment
- The Business of Veterinary Dentistry – Improving the Bottom Line
Lagomorphs and Rodents Chiroptera, Insectivores, Monotremes and Marsupials
Lagomorphs (rabbits) and rodents.
Introduction
Lagomorphs and rodents are increasingly becoming popular pets as well as an integral part of veterinary practice. These ‘pocket pets’ have a different anatomical and physiological make up compared to companion pets, such as dogs and cats. They also have a high incidence of oral problems that can be treated and managed by the veterinary surgeon.
The Order Lagomorph contains 66 species that are divided into two families: the Ochotoma (ie pikas) and the Leporidae (ie rabbits and hares). Pika are small 10-25cm mammals found in the Rocky Mountains of the USA and the steppes of central Asia (Clutton-Brock, 2001). The family Leporidae contain 52 species of rabbits and hares. (Harrison and Fowler, 1978). They are larger (25 – 75cm) than pikas and are found throughout the world, except Antarctica (Clutton-Brock, 2001). The word ‘rodent’ comes from the Latin verb ‘rodere’ meaning ‘to gnaw’. There are more than 100 species of rodents, (Crossley, 1995; Wiggs and Lobprise, 1990) including Old World rats and mice, squirrels, guinea pigs, chinchilla, capybara and beavers.
Until the mid 1900s, lagomorphs were considered to be a sub-order of rodents, because both have continuously growing incisor teeth. However, one of the main distinguishing features between lagomorphs and rodents is their dentition. All of these animals have their premolar/molar and the incisor teeth separated by a large diastema, no canine tooth and a long, narrow oral cavity. There are also differences in the incisor relationship and significant differences in their caudal dentition.
Oral examination
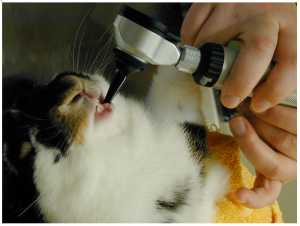
Lagomorphs and rodents have small mouth openings. A complete examination in a conscious animal is all but impossible. You should begin with an external examination. Palpate the ventral mandibular borders, the mandibular rami and the maxillary arches for swellings, pain, inflammation, tenderness and discomfort. Next check the incisor teeth for conformation, malocclusions and normal length. Then try to examine the premolar and molar teeth. It may be possible with a co-operative patient, but usually requires sedation or anaesthesia. Using an otoscope can give you an idea of what is happening in the caudal oral cavity, but general anaesthesia is required to complete the examination and perform any work.
Using an otoscope to examine the premolar and molar rabbit teeth.
I have used ketamine, opioids, and isoflurane for sedation and anaesthesia. Dr Tom Donnelly, a laboratory animal specialist from the Animal Medical Centre, in New York, USA and also based at the Chatswood Animal Hospital in Sydney, Australia prefers midazolam and ketamine in rabbits (personal communication), I have used the premed with buprenorphine followed by ketamine / medetomidine (University of Colorado doses) for guinea pigs, which give 20 – 30 minutes duration for dental surgery. This I have found to be ideal for incisor extractions or removing premolar / molar spurs, which is then reversed with Atipamezolw IM. The pocket pet anaesthetic regimes in referenced texts, see doses in table below. (Flecknell PA, 2000; Mason DE, 1997) (University of Colorado). Inhalation anaesthesia with Isoflurane is easily performed with a facemask or endotracheal tube.
Anaesthetic and analgesic dose rates.
| Rabbits | Guinea-pig | Rat | |
| Midazolam | 1 – 2 mg/kg IM, IV (light to moderate sedation) | 1 – 2 mg/kg IM, IV (light to moderate sedation) | 1 – 2 mg/kg IM (light to moderate sedation) |
| Medetomidine | 0.1 – 0.5 mg/kg SQ, IM (light-heavy sedation) | 0.3 – 0.5 mg/kg SQ, IM (light-heavy sedation) | 0.1 – 0.3 mg/kg SQ, IM (light-heavy sedation) |
| Atipamezole (Antisedan, Pfizer) | 1.0 mg/kg SQ (reverse Medetomidine) | 1.0 mg/kg SQ (5mg for every 1mg of medetomidine) (reverse Medetomidine) | 1.0 mg/kg SQ (reverse Medetomidine) |
| Atropine | 0.2-0.3 mg/kg IM, SQ | 0.05 mg/kg SQ | 0.05 mg/kg SQ |
| Glycopyrrolate | 0.01-0.02 mg/kg SQ | N/A | N/A |
| Ketamine | 20-50 mg/kg IM | 22-44 mg/kg IM | 22-44 mg/kg IM |
| Isoflurane | 3-5% (induction), 0.5-3% (maintenance) | 3-5% (induction), 0.5-3% (maintenance) | 3-5% (induction), 0.5-3% (maintenance) |
| Diazepam (mixed with ketamine) | 10 – 15 mg/kg (K) / 0.3 – 0.5 mg/kg (D) IM, IV | 20 – 30 mg/kg (K) / 1 – 2 mg/kg (D) IM | N/A |
| Butorphenol | 0.1 – 1.0 mg/kg IM, SQ, IV | 0.4 – 2.0 mg/kg SC | 1 -2 mg/kg SC |
| Ketamine/Medetomidine | N/A | 40 mg/kg (K) / 0.25 – 0.5 mg/kg (M) IM | N/A |
Applying a face mask to administer gaseous anaesthesia in a rabbit.
Once sedated, anaesthesia can be maintained by endo-tracheal intubation and maintenance with gaseous anaesthetic agents. Endo-tracheal intubation is difficult in rabbits, and all but impossible in smaller species, but can be achieved with practice or using an endoscope to visualise the trachea. Once placed, the endotracheal tube can be palpated in the trachea. To properly examine the premolar and molar teeth, the mouth must be held open. I usually use gauze tape over the incisor teeth and a buccal cheek pouch dilator.
Various sizes of cheek pouch dilators.
Opening the mouth using gauze looped over the maxillary and mandibular teeth and a buccal cheek pouch dilator.
The use of a speculum over the incisor teeth can be used for better visualisation, but I believe this places a lot of stress on the temporo-mandibular joints if it is over tightened, which is a common practice. Good lighting is also mandatory. A tongue depressor or a spatula can help to keep the tongue and buccal pouches out of view. A periodontal probe and explorer is indicated to assess tooth mobility and measuring periodontal probing depth.
A speculum. This design has two open loops (left end of picture) that are placed over the incisor teeth. The winding mechanism (on the right) is used to separate the loops.
Radiology is necessary to assess periapical pathology and root pathology. Most dental problems affect more than one tooth. Survey radiographs can be taken using periapical film and a dental radiograph machine. The small size of the oral cavity in most lagomorphs and rodents makes intra-oral films difficult to place, thus an extra-oral view may be required. The most useful views are rostro-caudal, dorso-ventral and lateral. If the film cannot be placed intraorally, the lateral view should be taken with a slight oblique angle to avoid superimposition of the mandibular tooth root apices. I find that oblique extra-oral views are easiest to take and produce good information.
Lagomorph Dental Anatomy
There has been some debate as to whether lagomorphs have a single set of teeth, but there has been data published, by Habermehl in 1975, that they have two sets of teeth, a deciduous set followed by a permanent set. The deciduous teeth are often shed prior to birth.
All incisor, premolar and molar teeth in the Lagomorphs are continuously growing and have no true root structure. They are termed aradicular hypsodont. Aradicular means no true root structure, whilst hypsodont means continuously growing. The permanent dental formula for the rabbit is 2 x (I 2/1, C 0/0, P 3/2, M 3/3) = 28 teeth in total.
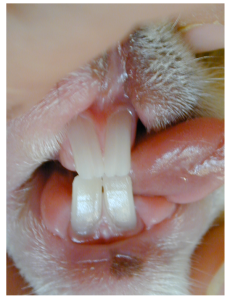
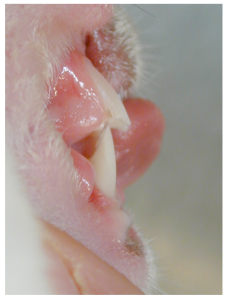
The lagomorph has two rows of incisor teeth in the maxilla, comprising 4 maxillary incisors in total. In the front row there are two large incisor teeth and positioned palatal to these are two smaller incisor teeth (or peg teeth). There are two mandibular incisors. The cusps of the mandibular incisor teeth are positioned and occlude between the large and peg incisor teeth.
Normal rabbit occlusion. Note the shape of the right maxillary incisor teeth, of which there is a large rostral and a small palatal incisor tooth. Note the shape and chisel edge of the single right mandibular incisor tooth. This is mirrored on the left side of the mouth.
Normal rabbit occlusion. Note the two maxillary incisor teeth and two mandibular incisor teeth. The smaller maxillary incisor ‘peg’ teeth are hidden behind the large incisors.
Normal rabbit occlusion. Note the position of the mandibular incisor edge just palatal to the maxillary incisor teeth with the mouth closed.
The lagomorph has three premolar and three molar teeth in the maxilla and two premolar and three molar teeth in the mandible bilaterally. The premolar and molar teeth have a relatively flat horizontal surface with transverse enamel folds. There are no canine teeth. Enamel is much thicker on the mesial (front) surface of the incisors and on the buccal (lateral) surfaces of the premolar and molar teeth, essentially forming a cutting blade. When the rabbit’s mouth is at rest, it is held in the midpoint of its forwards – backwards movement. The cutting surface of the premolar and molar teeth do not occlude at rest. In order to eat, the rabbit must bite off food into small pieces, which is performed by the incisor teeth. The sharp edges of the mandibular incisor teeth are drawn across the occlusal surfaces of the maxillary incisors in a slicing action. This action, due to the enamel edge acting as a blade, maintains the length of the incisor teeth, as the sharp enamel edge abrades the tooth and shortens it.
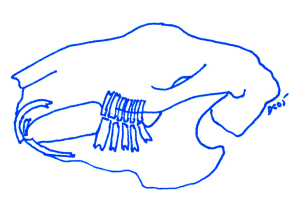
A rabbit skull showing the position of the teeth and relationship of the occlusion.
Diagram showing the jaw structure and dentition of the rabbit jaw in the resting position.
Both the premolar and molar teeth are maintained by the lateral chewing action, which brings the edges of the occlusal surfaces into contact. The left side mandibular and maxillary teeth work together, whilst the right side work together. During normal mastication, the rabbit chews on both sides of its mouth. As the rabbit chews the lingual edge of the mandibular premolar and molar teeth contacts the buccal edges of the maxillary premolar and molar teeth. The teeth are then moved across each other to the point where the palatal edge of the maxillary premolar and molar teeth contacts the buccal edge of the mandibular teeth. This action is repeated twice a second, depending on the type and nature of the food. If the rabbit eats commercially available food, such as pellets, the jaw movements are significantly shortened and the action produces less contact of the teeth, which in turn results in wear of the lingual side of the maxillary teeth and the buccal surface of the mandibular teeth. In turn this produces spurs and hooks on the buccal surface of the maxillary teeth and the lingual surface of the mandibular teeth.
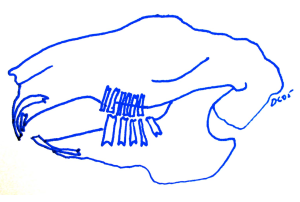
Diagram showing the position and relationship of dental occlusion during chewing with the premolar and molar teeth. Note the mandible is moved caudally (backwards), which takes the incisors out of contact.
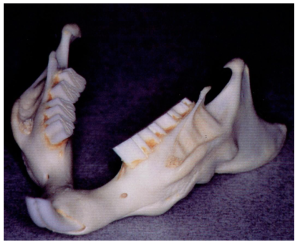
Diagram of cross section – rabbit premolar and molar overgrowth. Showing maxillary teeth growing towards buccal/lateral cheek and mandibular teeth growing lingually over the tongue.
Dental charts
Whilst the examination is being performed, a dental chart should be completed. This is usually a legal requirement, like taking and keeping a good history and recording medications, which have been prescribed. It is also good for comparisons when dental work is performed over multiple visits, multiple anaesthetics and time frames. The dental chart below is from Dr David Crossly (Manchester, UK), adapted by Dr Bob Wiggs (Dallas, USA) and the chart I use in the practice.
A rabbit dental chart by Dr David Crossley, Manchester, UK adapted by Dr Bob Wiggs, Dallas, USA.
Rodent dental anatomy
It is considered that rodents have no deciduous teeth and only one set of permanent teeth. Rodents have one row of incisor teeth in both the maxilla and mandible. They have two incisors in each row: four teeth in total. The incisors are termed aradicular hypsodont. The premolar and molar teeth are either hypsodont (guinea pig, chinchilla) or brachydont (rat, mouse and hamster). The dental formula varies between species, but four incisors, no canine teeth, few to no premolar teeth and a large diastema between the incisor and premolar or molar teeth are common features. The permanent dentition of the rodents are: guinea pig, chinchilla, capybara, porcupines, beavers: 2x (I 1/1, C 0/0, P 1/1, M 3/3) = 20 teeth in total, rat and mice: 2x (I 1/1, C 0/0, P 0/0, M 3/3) = 16 teeth in total, squirrel: 2x (I 1/1, C 0/0, P 1-2/1, M 3/3) = 20 – 22 teeth in total and hamster: 2 x (I 1/1, C 0/0, P 0/0, M 2-3/2-3) = 12 – 16 teeth in total.
All rodents ‘gnaw’ and have a wide range of movement in the temporo-mandibular joint, compared to the lagomorphs. At rest the incisors of the rodents are out of occlusion.
Tooth growth and the periodontal ligament
Rodent incisors grow at approximately 1mm per week. Lagomorphs maxillary incisors grow at 2mm per week and the mandibular incisors grow at 2.4mm per week (Wiggs and Lobprise, 1995).
The periodontal ligament in the hypsodont teeth has a different structure to the ligament of brachydont teeth. In brachydont teeth, the periodontal ligament is continuous and spans the space between the tooth root cementum and the alveolar bone. In the hypsodont tooth, the periodontal ligament has two sets of collagen fibres that attach to either the alveolar bone or cementum, but not both. The fibres from each side then attach to each other.
Signs of dental disease
Common clinical signs associated with dental disease:
- Decreased food intake
- Weight loss
- Poor coat condition
- Change in defecation
- Salivation, wet dewlap
- Ocular and nasal discharge
- Mandibular swelling
- Difficulty closing the mouth
- Elongated incisor teeth
- Facial swelling
- Systemic disease
- Death
Malocclusion
Lagomorph and rodent incisors continually grow and therefore any disruption in the usual attrition or wear will result in malocclusion. In lagomorphs, incisor malocclusion can be either primary or secondary in nature.
Primary incisor malocclusion
Primary malocclusions occur in rabbits under 12 months of age. Rabbits as young as three weeks of age may show brachygnathism of genetic origin resulting in a malocclusion and overgrowth of the mandible. An autosomal recessive gene for a shortened maxillary diastema is the probable cause (Fox and Crary, 1971). It is commonly seen in dwarf rabbits. This causes a longer mandible and an incisor malocclusion. The incisors continue to grow and with no attrition or wear, the maxillary incisors tend to curl and twist in the oral cavity, and the mandibular incisors grow laterally, appearing similar to an elephant or pig tusk. This often results in an inability of the rabbit to eat and prehend food so they slowly lose body condition, slobber, drop food from the mouth and suffer moist dermatitis of the skin around the mouth and on the chest.
In rabbits and rodents with continuously growing premolar and molar teeth, incisor malocclusions may lead to secondary premolar and molar issues. When the incisors are unable to section food into bite size pieces, the pet does not chew on the premolar and molar teeth properly and therefore they do not wear adequately. The overgrowth of these teeth force the mouth open and present the previously described clinical signs. If the maxillary premolar and molar teeth overgrow, they grow laterally, lacerating the buccal mucosa, whilst the mandibular premolar and molar teeth grow lingually, entrapping the tongue.
Secondary incisor malocclusion
Secondary incisor malocclusion occurs in older pets, usually over 12 months of age. This is more commonly diagnosed than primary incisor malocclusions. Secondary malocclusion may result from lack of attrition and wear of both the incisor teeth or the premolar and molar teeth. This may be due to general illness, lack of chewing, a change of diet, oral infections, temporomandibular joint dysplasia or tooth fractures.
Premolar and molar malocclusions
Premolar and molar teeth abnormalities and malocclusions are common in both the lagomorph and guinea pig. Primary overgrowth of the premolar and molar teeth in lagomorphs and guinea pigs, with secondary overgrowth of the incisors is a common occurrence. The majority of the incisor malocclusions in the rabbit are secondary to premolar and molar teeth overgrowth. It has also been reported due to excessive selenium intake (Williams, 1976). Although calcium and Vitamin D deficiency may be involved in the aetiology (Harcourt-Brown and Baker, 2001), the primary cause is thought to be feeding diets that provide inadequate abrasion (Crossley 1995, Redrobe 1997). A large number of cases of molar malocclusion in guinea pigs is related to Vitamin C deficiency. Thought to weaken the periodontal ligament and allow excessive movement of the tooth, leading to lack of chewing, reduced lateral movement and production of hooks and spurs. Treatment, a change in diet and prevention by supplement with Vitamin C powder 30-50mg per day or add in parsley, green capsicum or oranges.
The first step in treatment of incisor malocclusion is to determine whether you have a primary or a secondary incisor malocclusion.
Diagnosis
It is unusual to diagnose premolar and molar teeth malocclusions in the early stages, as I find clinically the majority of pets are presented well into the course of the disease. Often they are past the point when treatment can be successful. This is an important point, as many owners have very high and often unrealistic treatment expectations and outcomes. Often the pet needs to be euthanased.
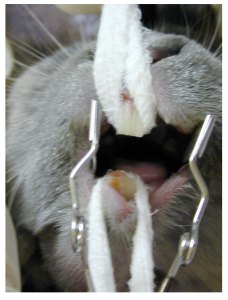
In the consulting room in the awake pet, a general intra-oral examination can be performed using an otoscope. If the 5mm diameter scope is used, the height of the premolar and molar teeth can be estimated quite easily. On oral examination, in the rabbit, the teeth should have a flat occlusal surface. The maxillary premolar and molar teeth are almost level with the gingiva and the mandibular premolar and molar teeth should be 1-2mm above the gingiva. Once overgrowth occurs, the teeth become longer, the maxillary premolar and molar teeth grow laterally, flare buccally, produce spurs and hooks and ulcerate the mucosa, whereas the mandibular premolar and molar teeth grow lingually over the tongue. A complete examination though must be performed under general anaesthesia.
An oral examination using gauze and a cheek pouch dilator allows visualisation of the overgrown right sided mandibular premolar and molar teeth.
In the guinea pig, the mandible is wider than the maxilla and the maxillary cheek teeth lean buccally and the maxillary cheek teeth lean lingually. The occlusal plane is approximately 30 degrees to the horizontal. It is very difficult to examine the guinea pig oral cavity completely with the pet awake. The otoscope can be used as previously described. There has been an association between dental problems and hypovitaminosis in the guinea pig (Klaus and Bennett 1999, Brown and Rosenthal 1997). Hypovitaminosis leads to collagen defects and a weaker periodontal ligament, resulting in tipping and movement of the tooth in the socket. Unfortunately the pets are often presented when the disease is well advanced and treatment options are limited. Primary incisor malocclusion in guinea pigs is rare, the majority of incisor malocclusion is due to cheek teeth overgrowth.
The normal mandible and teeth of the guinea pig. Note the 30 degree lingual angle of the premolar and molar teeth.
Traumatic tooth injuries
Pets chewing on cages or other inappropriate objects may result in tooth fractures and trauma. But, the most common traumatic tooth injury I see is following the use of toenail clippers used to shorten incisor teeth. The result of using nail clippers is shattering of the tooth and oblique fractures, which often extend subgingivally and pulpal exposure. Once the pulp is exposed, inflammation and infection occur, the pet stops eating, abscessation results and endodontic treatment is required.
Progression of acquired dental disease
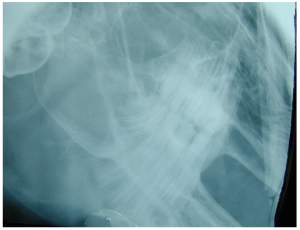
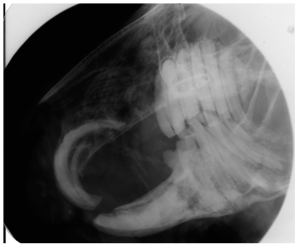
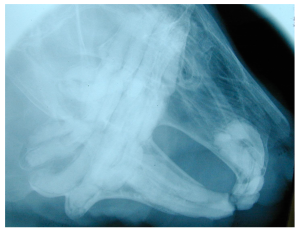
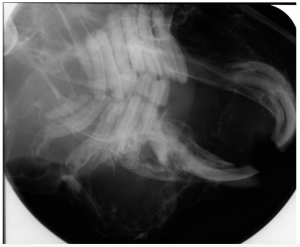
Frances Harcourt-Brown, an English veterinarian with an interest in rabbits has written many papers on dental conditions and has developed a grading system for rabbit dental disease. She found that most of the literature was based on laboratory and commercial rabbits, although most veterinarians treat pet rabbits. She has described the following clinical and radiographic signs for pet rabbits:
Grade1:
- Smooth ventral border to the mandible
- Lack of elongated tooth subgingivally
- Normal bone skull density
- Parallel smooth linear pattern of premolar and molar tooth subgingivally
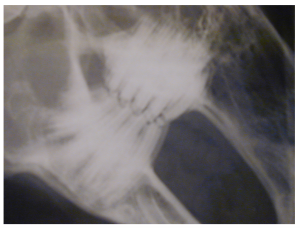
Radiograph showing Grade 1 disease.
Grade 2
- Elongation of tooth subgingivally
- Early tooth divergence from parallel array
- Hard bone swellings at site of periosteal penetration along the mandible
Radiograph showing Grade 2 disease.
Grade 3
- Deviation of teeth from normal parallel array
- Loss of normal occlusal zigzag pattern
- Loss of supporting bone and change in bone or tooth position
- Clinically signs include difficulty grooming, tongue lacerations, salivation, drooling, abscess formation, tooth mobility
Radiograph showing Grade 3 disease.
Grade 4
- Cessation of tooth growth
- Broken crowns and dysplastic appearance of teeth
- Loss of linear pattern
- Blurred dental outline due to enamel loss
Radiograph showing Grade 4 disease.
Grade 5
- End stage disease
- Abscesses, osteomyelitis, mobile teeth
- Difficulty in differentiating one tooth from another
- All premolar and molar teeth affected
- Usually infection of tooth with osteomyelitis
Radiograph showing Grade 5 disease.
Stephen Hernandez-Divers has used Frances Harcourt-Brown’s radiographic findings and correlated them to clinical success. He found that rabbits with Grade 1 and 2 lesions have an 80% chance of successful dental treatment, whilst rabbits with Grade 4 and 5 lesions have a 15% chance of treatment success.
Periodontal disease that occurs in other species does not typically occur in lagomorphs and rodents. When periodontal disease and the secondary sequelae such as gingival tissue infection, facial and jaw abscesses, endodontic infection, penetrating foreign bodies do occur, they can be treated as they would in other species. A more commonly seen disease in the rabbit is a periapical abscess or osteomyelitis. Soft tissue abscesses or bony abscesses are usually a result of penetrating bacterial infection, often due to Pasteurella sp. and Fusobacterium necrophorum. Soft tissue abscesses should be treated by complete resection, whereas bony involvement should be curetted and antibiotics placed.
Treatment of dental problems
The primary role of treatment is to remove the malocclusion, provide occlusal adjustment (odontoplasty), extract affected teeth and complete abscess debridement.
Odontoplasty (crown height reduction)
The aim of tooth trimming is to recreate normal occlusion, where possible, or relieve a malocclusion and thus the resultant trauma. Tooth trimming, thereby reducing the height of the tooth, is ideally performed under general anaesthesia. Whilst reduction of the crown height of the incisor teeth can be performed with the pet awake or sedated, reducing the height of the cheek teeth must be performed under general anaesthesia. Thermal damage to the tooth can be reduced with a water spray but anaesthesia and endotracheal intubation is needed to avoid drowning the animal.
Incisors
Traditionally, incisor teeth have been trimmed with toe nail clippers. Trimming of the incisors with nail clippers designed for cutting dogs’ toe nails should be avoided to prevent fracturing the tooth and exposing the pulp (Wiggs and Lobprise 1990, Lobprise and Wiggs 1991). With the availability of high speed dental equipment, i.e. a high speed drill and bur, I would consider it unacceptable and malpractice to use toe nail clippers. A high speed diamond bur can be used to reduce the tooth height. The correct angle of occlusion should be maintained. To protect the surrounding soft tissues, a tongue depressor can be placed behind the incisor to protect the tongue. Dremel tools should not be used since they have electrocuted veterinarians and animals in the USA.
If the incisor tooth fractures during trimming or the height is reduced too severely, the pulp may be inadvertently exposed. The pulp should be treated by direct pulp capping immediately the pulp is exposed. The tooth should be sterilised with 0.05% chlorhexidine, approximately 2mm of coronal pulp is removed with a # 1 round diamond bur in a water cooled high speed handpiece. Haemorrhage is controlled with a sterile paper point, followed by sealing of the pulp, which requires placement of a hard setting calcium hydroxide cement (e.g. Life) followed by an intermediate restorative material (e.g. IRM) (Wiggs and Lobprise 1995). The opposing teeth may need to be trimmed. The trauma may result in damage to the pulp tissue and necrosis of the tooth so it may cease to grow or grow in an abnormal position. The hard setting composites should not be used as they do not wear like a normal tooth.
A common problem seen in guinea pigs occurs when owners and veterinary staff trim mandibular incisor teeth too short, the guinea pig is unable to eat and they starve to death. This occurs as the incisor teeth are longer in guinea pigs compared to rabbits.
Premolars and Molars
In order to trim the premolar and molar teeth, the correct equipment and instruments are required. Good lighting, a mouth gag or gauze tape, cheek dilators, tongue retractors, slow speed handpiece, HP fissure burs, a bur guard, rasp or file are required.
To access the cheek teeth, the mouth must be opened. The incisor teeth can be moved apart. Whilst a gag will do this, often too much pressure is applied to the temporomandibular joint, so I tend to use an assistant with two pieces of gauze bandage looped around the maxillary and mandibular incisor teeth. A cheek dilator can be used to push the cheek pouches out of the view of the teeth. A flat spatula is used to move the tongue out of the way. Ronjeurs, molar cutters and slow speed burs are introduced into the mouth to trim the teeth now that the operator can visualise what they are doing.
My preference to trim the premolars and molar teeth is to initially use a pair of molar cutters designed for the procedure. The molar cutters remove a large piece of tooth atraumatically and quickly. I then smooth the rough edges with a hand-held file and a slow speed bur with a bur guard attached. The guard reduces the chance of soft tissue trauma as it prevents the HP bur from contacting the soft tissues whilst it is being used against the tooth surface. The tissue caudal to the last molar bleeds easily if traumatised and may result in death of the pet.
Molar cutters.
Molar cutters used to trim lingual hooks and spurs from the mandibular premolar teeth.
Hand file or rasp.
A hand file is used to smooth the premolar teeth.
Rodent bur guard and HP bur in a straight nose-cone attachment.
The choice of bur depends on the size of the pet. A #701 or #702 tapered fissure tungsten carbide is a good choice. Ideally, the bur should have water to cool it, but the pet should not be drowned!! A bur will blunt in a few seconds on contact with the tooth enamel without water. It is ideal to apply the bur for one second, take it off for two seconds, and repeat. Another option is to use a dry bur and have an assistant drip water onto the tooth.
When the teeth are trimmed, the normal occlusal angle of the premolar and molar teeth should be maintained, which is horizontal in rabbits and 30 degrees to the horizontal in guinea pigs. The oral cavity should be cleaned after trimming of any chips or pieces of tooth to avoid aspiration. Occlusion should be checked by laterally moving the jaw and feeling and listening for clicking and resistance. If the teeth have been severely overgrown, the masseter muscles will have been stretched, so a liquid diet may need to be fed for 3-4 days until the pet resumes normal eating.
Extractions
An alternative to tooth trimming is extraction. Extraction may be performed when a tooth is involved in an abscess complex, when it is suffering from periodontal disease making the tooth mobile and no longer a functional entity, or when the problem is an incisor malocclusion, the pets’ owners are tired of having the incisor teeth trimmed every two weeks.
Performing extractions with safety requires using a general anaesthesia and ideally an endotracheal tube. Nasal placed intubation or a mask to administer gaseous anaesthesia placed over the pet’s nose can be used as these allow a greater working space in the oral cavity. Specialised instruments are required to perform extraction cleanly. A luxator, curved root elevator, molar extraction forceps, Crossley luxators, Molt #2 (EX-9) periosteal elevator are a good start. In rats and guinea pigs, an 18 gauge needle can be used.
In combination with anaesthetic drugs, nerve blocks should be placed to provide analgesia. Incisor teeth are readily accessible and therefore considered to be the easiest to extract. A closed technique can be used to extract the incisor teeth. In small pets, i.e rats, an 18 gauge needle can be used to break down the periodontal ligament. In the rabbit, a Crossley luxator and Molt #2 (EX-9) elevator can be used. The portion of the tooth located subgingivally is exceptionally long in both the maxillary and mandibular incisor teeth. The maxillary incisor has a tight curve on the subgingival portion of the tooth, similar to a circle with a 5cm diameter, whereas the mandibular tooth has a milder curve, similar to a circle with a 10cm diameter.
Initially the oral cavity should be sterilised with a 0.05% chlorhexidine solution. The first step is to sever the epithelial attachment using a Molt #2/#4 periosteal elevator or a #11 scalpel blade. The blade is inserted into the gingival sulcus and the attachment is severed from the underlying alveolar bone ridge from around the entire circumference of the tooth. In small rodents, an 18 gauge needle can be used, in lagomorphs or guinea pigs, a Molt #2/#4 (EX-9), or Crossley luxator can be utilised. The instrument is inserted into the space between the tooth and the bone occupied by the periodontal ligament.
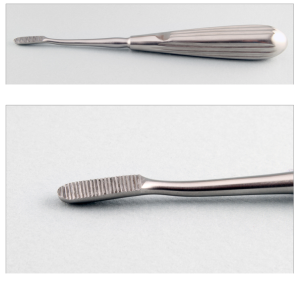
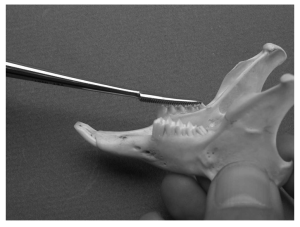
A Crossley incisor elevator.
A Molt #2/#4 (Cislak EX-9) periosteal elevator.
A curved deciduous tooth elevator can be used on the rostral and palatal/lingual surfaces, whereas the Molt #2/#4 or Crossley can be used on the buccal or mesial surfaces. After the instrument is placed, a slight twisting motion parallel to the tooth is used to break down the periodontal ligament. The elevator is slowly advanced apically and around the circumference of the tooth. Once loose the tooth can be grasped with molar extraction forceps and removed.
A curved deciduous elevator.
The use of early traction, leverage or torque in excess of what is needed, may break the tooth. If the tooth breaks, the remains should be removed. If left, the tooth will regrow and you will have another opportunity to remove it. Once the tooth is removed, the socket should be flushed and curetted of debris and granulation tissue, and any bony spicules smoothed. The sulcus is closed by suturing the gingiva with an absorbable suture. My preference is 3/0 Vicryl. Catgut causes a severe inflammatory reaction in rabbits and rodent. Hard sutures, such as PDS, result in sharp pointed ends that irritate and inflame the local tissues. The pet should be placed on antibiotics and analgesia, fed a soft diet, liquid apple or carrot juice, until the pet eats normally.
Extraction of the premolar and molar teeth is difficult due to lack of access. In rats and mice, extraction is difficult because of their small size, whereas in lagomorphs and guinea pigs they are difficult due to the long thin open ended root. Approaches to extraction include, the intra-oral non-surgical, extra-oral surgical and the buccal approach. The most common indication for extraction of premolar and molar teeth are endodontic complications, periodontal diseases or periapical abscessation.
In rats and mice, the cheek teeth have long thin roots and are best extracted using a 20 gauge needle attached to a 10cc syringe. Bend the tip of the needle and use it as a pick, slowly breaking down the periodontal ligament. The teeth often break, unless the tooth is already loose, and it is almost impossible to retrieve a root tip without causing excessive trauma.
Extraction of lagomorph and guinea pig teeth are easier to extract as they are often mobile. The same technique is used, along with the addition of right-angles periosteal elevator and molar extraction forceps. The periosteal elevator is forced into the periodontal space to sever the ligament and push the tooth away for the bone. One the tooth is mobile it can be gently rocked from side to side or in a figure of 8 pattern until it loosens and can be removed from the socket.
A Crossley right-angle periosteal elevator.
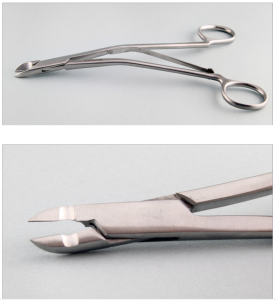
Molar extraction forceps.
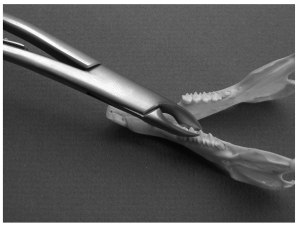
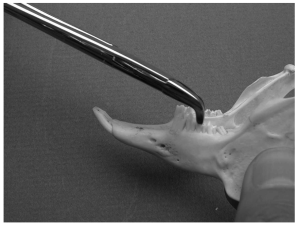
Molar extraction forceps used to grasp and remove a premolar from a rabbit mandible.
Extraction of one tooth from each arcade generally does not affect the overgrowth of the occlusion, but more than this will result in uneven wear and the need for more frequent odontoplasty.
Extraction using a buccotomy, incising the cheek from the commissure caudally, to gain access makes the extraction easier but post operative care is often worse than the initial dental problem. I would not advise this technique unless it is a last option and post-operative care is guaranteed to be outstanding.
When the premolar and molar tooth is not mobile, there is inadequate space to obtain access, or an abscess is present, then an extra-oral approach can be used. Often the area of interest can be easily found as the abscess is palpable on the ventral border of the mandible or the abscess has ruptured and there is a draining sinus. An incision is made over the abscess, which is best determined by radiographs, and the bone is either removed with a high speed bur or curetted with a hand held periosteal elevator to leave an open cavity. The tooth can be pushed into the oral cavity and subsequently extracted. A follow-up radiograph will show whether the bone has been adequately curetted. The intra-oral site should be sutured with an absorbable suture material, the sulcus filled with an osteogenic product or Doxirobe gel. The ventral incision site is closed.
Treatment of abscesses
Abscesses involving extensive amounts of osteomyelitis can be generally assumed to be difficult to treat, but it worth trying before the rabbit is euthanased, as palliative care can often be achieved. Soft tissue abscesses are best treated by complete resection of the tissue. If the abscess cannot be completely removed then alternative treatment includes curettage of all affected tissue and extraction of all teeth affected in the area. Initial opening and curettage of the bony area and placement of medication is performed. Based on radiographic findings, the bony spurs and excessive bone production must be removed. Often the abscess grows and bony channels form which aid in protecting and preventing complete removal.
There are a number of treatment options that have been used with a various degree of success. One method is to repeatedly inject antibiotics into the abscess capsule. This has been shown and is correlated with variable success. Another method is to pack the abscess cavity with calcium hydroxide paste (Remeeus and Verbeek, 1995). Calcium hydroxide is alkaline with a pH of 13, and thus is very caustic and can result in bone and tissue necrosis. Despite a reported high success rate, I have found the calcium hydroxide leaks into the surrounding tissues and causes severe muscle necrosis and tissue sloughing. I would therefore not recommend this treatment option.
Another treatment option is to pack the cavity with antibiotic impregnated methylmethacrylate (AIPMMA) beads, which has shown some promise. The advantage of beads is the slow release of the antibiotic into the surrounding cavity and tissues. The beads are exothermic, so a heat resistant antibiotic must be chosen, such as gentamycin, tobramycin or cephazolin. Once you add the antibiotic to the mixture, you must work fast, or the bead mix will set before you roll the PMMA into individual beads. The abscess must be well excised and 100% of the pus removed prior to placement of the beads.
A newer treatment, which both David Crossley and I have used with some success, is to pack the curetted abscess area with Doxirobe, a doxycycline impregnated polylactic acid gel, which degrades over weeks and releases doxycycline into the area. I have had one rabbit which following placement of Doxirobe has been abscess free for 18 months. Crossley has reported success in five out of six rabbits, with one being controlled for over 11 months (Crossley, 2003). It is extremely important to completely curettage out any affected and diseased bony lesions, pus and diseased tissue from the abscess.
Often the area of interest can be easily found as the abscess is palpable on the ventral border of the mandible or the abscess has ruptured and there is a draining sinus. An incision is made over the abscess, which is best determined by radiographs, and the bone is either removed with a high speed bur or curetted with a hand held periosteal elevator to leave an open cavity. The tooth can be pushed into the oral cavity and subsequently extracted. A follow-up radiograph will show whether the bone has been adequately curetted. The intra-oral site should be sutured with an absorbable suture material, the sulcus filled with an osteogenic product or Doxirobe gel. The ventral incision site is closed.
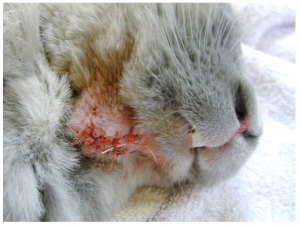
A rabbit with a draining abscess on the ventral border of the mandible.
Post-surgery, sutured and recovering.
Doxirobe antibiotic gel.
Once the rabbit has a Grade 4 or 5 dental disease, I have found that they end up being placed on either long life antibiotics such as Baytril or Doxycycline, or they are euthanased. Dosages: Baytril 15mg/kg sid for rabbits and 10 mg/kg for guinea pigs and rats or doxycycline 2.5mg/kg sid. Pulse therapy where the pet is given antibiotics of one week on and three weeks off, can be an alternative to continuous antibiotics.
Chiroptera (bats), insectivores, monotremes and marsupials.
Covered in the published paper section – Oral biology and disorders of chiroptera, insectivores, monotremes, and marsupials. The Veterinary Clinics of North America. Exotic Animal Practice – Oral Biology, Dental and Beak Disorders 6 (2003) 523-564.
References
Brown SA , Rosenthal KL. Self Assessment Colour Review of Small Mammals. London, UK: Manson Publishing, 1997, p. 77-78.
Clarke DE. Oral biology and disorders of chiroptera, insectivores, monotremes, and marsupials. Vet Clin Exot Anim 6 (2003) 523-564.
Clutton-Brock J. Mammals. In: Burnie D, ed. Animal. London: Dorling Kindersley; 2001. p. 141-3.
Crossley DA. Clinical aspects of rodent dental anatomy. J Vet Dent; 1995, 12(4):131-135.
Crossley DA. Clinical aspects of lagomorph dental anatomy: the rabbit (Oryctolagus cuniculus). Journal of Veterinary dentistry, 1995, 12(4):137-140.
Crossley DA. Oral Biology and disorders of lagomorphs. In: Oral biology, dental and beak disorders. Crossley DA, ed. Saunders, Philadelphia. Vet Clin Exot Anim 6 (2003) 629-659.
Divers SJ, Lawton MPC. (1999). Antibiotic-Impregnated Polymethylmethacrylate Beads as a Treatment for Osteomyelitis in Reptiles. 1999 Proceedings, Asociation of Reptilian and Amphibian Veterinarians
Flecknell PA. Anaesthesia. In: Flecknell PA, ed. Manual of rabbit medicine and surgery. Gloucester (UK): BSAVA, 2000. p. 103-16.
Fox RR, Crary DD. Mandibular prognathism in the rabbit: Genetic studies. J Hered 1971; 62:23.
Habermehl KH. Die Altersbestimmung bei Haus – u. Labortieren. 2nd ed. Berlin: Verlag Paul Parvey; 1975.
Harcourt-Brown FM, Baker SJ. Parathyroid hormone, haematological and biochemical parameters in relation to dental disease and husbandry in rabbits. Journal of Small Animal Practice. 2001. 42(3):130-136.
Harrison GJ, Fowler ME. Rabbits, hares and pikas. In: Fowler ME, ed. Zoo and wild animal medicine. Philadelphia: WB Saunders; 1978. p. 481-90.
Klaus P, Bennett RA. Management of abscesses of the head in rabbits. Proceedings of the North American Veterinary Conference, Orlando, USA, 1999.
Lobprise HB, Wiggs RB. Dental and oral diseases in lagomorphs. J Vet Dent 1991; 8 (2):11
Mason DE. Anaesthesia, analgesia and sedation for small animals. In: Hillyer EV, Quesenberry KE, eds. Ferrets, rabbits and rodents. Philadelphia: WB Saunders Company; 1997. p. 378-91
Redrobe S. Surical procedures and dental disorders. In: Flecknell P, ed. Manual of Rabbit Medicine and Surgery. Cheltenham, UK:BSAVA, 1997 p. 129-133.
Remeeus PGK and Verbeek M. The use of calcium hydroxide in the treatment of abscesses in the cheek of the rabbit resulting from a periapical disorder. J Vet Dent 1995; 12: 16-22.
Wiggs RB, Lobprise HB. Dental disease in rodents. J Vet Dent 1990; 7(3):6-8.
Wiggs RB, Lobprise HB. Prevention and treatment of dental problems in rodents and lagomorphs. In: Crossley DD, Penman S eds. Manual of Small Animal Dentistry. Gloucestershire, UK: British Small Animal Association; 1995:84
Wiggs RB, Lobprise HB. Dental and oral disease in rodents and lagomorphs. In: Wiggs RB, Lobprise HB, eds. Veterinary Dentistry. Practice and Principles. Philadelphia: Lippincott; 1997. p. 525.
Williams CSF. Practical Guide to Laboratory Animals. St. Louis, MO: CV Mosby Co; 1976:149.