Introduction to Anaesthesia
Member Only Content
Members Links
Printer Articles
- Anatomy
- Dental Extractions of Single Rooted Teeth
- Direct Pulp Capping (Partial Coronal Pulpectomy)
- Extractions
- Feline Dental Radiography
- Homecare
- Infra-orbital nerve block in the dog.
- Introduction to Anaesthesia
- Members Lectures
- Paedodontics
- Periodontal Disease 3 – Saving the Periodontally Diseased Tooth
- Periodontology
- Pocket Pets and Exotics
- Procedure Videos
- Reference Manuals
- Rest acid etch
- Restoratives – Composite Bonding
- Setting Up a Dental Surgery – Dental Equipment
- The Business of Veterinary Dentistry – Improving the Bottom Line
Introduction to anaesthesia
In recent years, the awareness among veterinarians has increased on the need to provide comprehensive pain relief, but there are still several reasons why dental pain in dogs and cats may go untreated (Carroll, 1999; Gayner, 1999; Hendrix and Hansen, 2000; Lascelles, 1999), including:
The belief amongst some veterinarians that dogs and cats do not feel pain in the same way as humans. Veterinary staff not recognising the clinical signs of dental pain.
Veterinarians being concerned about the cost of analgesic drugs or the side effects associated with them.
The belief amongst some veterinarians that giving pain relief may lead to self trauma and further tissue damage.
Pain evoked during dental procedures in humans has been equated to other painful surgical procedures in humans. The same could be true in dogs and cats, where pain and inflammation during dental procedures follow the same physiological pathways as other painful procedures (Barnett, 1997). Clarke (2004) found that the administration of pre-emptive analgesia had a significant effect on the reduction of pain when compared to when no analgesia was administered in dogs undergoing surgical tooth removal.
Definition of anaesthesia
Local anaesthesia has been defined as a loss of sensation in a circumscribed area of the body caused by a depression of excitation in nerve endings or in an inhibition of the conduction process of the peripheral nerves (Covino and Vassallo, 1976). An important feature of local anaesthesia is that it produces this loss of sensation without inducing a loss of consciousness.
Pain and the generation of impulses
The discovery in the late 1800s of a group of chemicals with the ability to prevent pain without inducing a loss of consciousness was a major step forward in the dentistry profession. The concept behind the actions of local anaesthetics is simple – they prevent both the generation and conduction of a nerve impulse. Therefore the source of the impulse is prevented from reaching the brain, and is thus not interpreted as pain by the patient.
The function of a nerve is to carry electrical action potentials. Action potentials are transient depolarisations of the membrane and can be initiated by thermal, chemical, mechanical or electrical stimuli. Once an impulse is initiated, its duration and intensity remains steady until it reaches the end of its length. A nerve possesses a resting potential of –70 mV that exist across the nerve membrane, producing a differing concentration of ions on either side of the membrane. The interior is negative relative to the exterior.
A stimulus excites the nerve, which initiates a slow depolarisation within the nerve. At a critical level, an extremely rapid phase of depolarisation occurs termed the threshold potential, which results in a reversal of the electrical potential across the nerve membrane and a +40 mV potential exists on the interior of the nerve cell (de Jong and Wagman, 1963). After depolarisation, repolarisation occurs and the nerve returns to its original resting state. All this occurs in 1 millisecond – depolarisation (0.3 msec) and repolarisation (0.7 msec).
At rest, K+ remains within the nerve membrane, Cl- remains outside the nerve membrane. Na+ is greater outside the nerve membrane and the fact that the membrane is only slightly permeable to Na+ keeps it outside. Depolarisation leads to an increase in permeability of the cell membrane to Na+ ions, due to widening for ion channels sufficient to permit unhindered passage of hydrated Na+ ions. The rapid influx causes depolarisation of the nerve membrane from its resting level. Once the potential decreases 15 mV, membrane permeability to Na+ increases dramatically and Na+ ions rapidly enter the axoplasm. The permeability to K+ also increases resulting in an efflux of K+. The movement of Na+ outward and K+ inwards during repolarisation occurs passively.
Action of local anaesthetics
Local anaesthetics interfere with the transport of action potentials in the following ways:
- Altering the basic resting potential of the nerve membrane
- Altering the threshold potential
- Decreasing the rate of depolarisation
- Prolonging the rate of repolarisation
Local anaesthetics primarily have an effect on the depolarisation phase of the action potential (de Jong and Wagman, 1963). The nerve membrane is the site where local anaesthetics exert their pharmacological action. There have been many theories on how they work, but the specific receptor theory is the most favoured (Strichartz and Ritchie, 1987), where they act by binding to specific receptors on the sodium channel. The action of the drug is direct, and once the drug has gained access to the receptor, permeability to sodium ions is decreased or eliminated and nerve conduction is interrupted.
Types of anaesthetics
Local anaesthetics can be classified as either amino esters or amino amides. The esters (procaine) are readily hydrolysed in aqueous solution. The most commonly available anaesthetics in veterinary dentistry are the amides (lignocaine, mepivacaine, prilocaine and bupivacaine) and are relatively resistant to hydrolysis. The pH of the tissues greatly affect the nerve-blocking action. Inadequate anaesthesia results when injecting into an inflamed or infected area. Normal tissue pH is 7.4, whereas the pH of an inflamed area is 5.0 to 6.0. After administration of a local anaesthetic into the soft tissues, molecules of the local anaesthetic transverse the distance from one site to another along the concentration gradient.
The timing of administration of the local anaesthetic – ‘Pre-emptive analgesia’
In human dentistry, the use of local analgesia in conjunction with general anaesthesia is a common treatment regime. The timing of analgesic administration is important, as anaesthesia does not necessarily equate with analgesia. It has been shown that administration of analgesics before infliction of pain, termed ‘pre-emptive analgesia’, greatly reduce the degree of pain the central nervous system registers, and therefore post-operative pain (Woolf and Wall, 1986; Wall, 1988; Woolf, 1989; Clarke, 2004). The whole concept of ‘pre-emptive analgesia’ rests on the belief that one is blocking sensory nociceptive information from onward transmission. If nociceptive information is permitted to reach the spinal cord a state of central hypersensitivity or ‘wind-up’ is induced.
The Armamentarium
The most common syringe used is the dental aspirating syringe and represents the standard of care. The equipment has three components: the needle, the syringe and the cartridge. If the needle tip rests within the lumen of a blood vessel when a negative pressure is applied to the thumb ring, blood enters the needle lumen and is visible in the cartridge. There are a number of different gauges of needle as well as length. The most commonly used needles are 27-gauge 36mm (long) and 30-gauge 10mm (short) (Malamed, 2004). The dental cartridge is a glass cylinder containing local anaesthetic. The cartridge contains 2.2mls of anaesthetic solution. If a dental aspirating syringe is not available then a 27 gauge hypodermic needle and syringe may be used.
A self aspirating dental syringe.
Types of analgesics
The most common anaesthetics used for local analgesia in veterinary dentistry are: bupivacaine, lignocaine, mepivacaine and prilocaine.
The dose of local anaesthetic drugs are presented in mg/kg. To increase safety, one should always use the minimium drug doses and the smallest clinically effective dose. Maximum doses are unlikely to be reached in large breed dogs, but it is very easy to overdose the small breed dogs and cats. The maximum calculated drug dose should always be decreased in medically compromised or older patients. Changes in liver function, plasma protein binding, blood volume, and other important physiological functions influence the manner in which local anaesthetics are distributed and biotransformed in the body (Iwatsubo et al, 1997).
| Lignocaine | Mepivacaine | Prilocaine | Bupivacaine | |
| Formula | 2-Diethylamino 2’,6-acetoxylidide hydrochloride | 1-methyl 2’,6’-pipecoloxylidide hydrochloride | 2-Propylamino-o-propionotoluidide hydrochloride | 1-Butyl-2’,6’-pipecoloxylidide hydrochloride |
| Metabolism | Liver (microsomal fixed function oxidases) | Liver (microsomal fixed function oxidases) | Liver (hepatic amidases) | Liver (hepatic amidases) |
| Excretion | Kidneys (10% unchanged) | Kidneys (1% – 16% unchanged) | Kidney | Kidney (16% unchanged) |
| Vasodilation | Yes | Slight | Yes | Significant |
| pH (plain) | 6.5 | 4.5 | 4.5 | 4.5-6.0 |
| Onset of Action | Rapid (2 to 3 minutes) | Rapid (1.5 to 2 minutes) | Slower (2 to 4 minutes) | Slow (6 to 10 minutes) |
| Half-life | 90 minutes | 1.9 hours | 1.6 hours | 2.7 hours |
| Max recommended dose | 4.4 mg/kg | 6.6mg/kg | 6.0 mg/kg | 1.3 mg/kg |
| Maximum dose | 1 cartridge per 10kg bodyweight | 1 cartridge per 10kg bodyweight | 1 cartridge per 10kg bodyweight | 1 cartridge per 10kg bodyweight |
| DOA (plain) (soft tissue) | 30 minutes | 2 to 3 hours | 2 to 4 hours | 6 to 8 hours |
| DOA (soft tissue) (with vasoconstrictor) | 3-5 hours | 3 to 5 hours | 5 to 8 hours | 8 to 12 hours |
Properties of the four commonly used local anaesthetic agents.
Lignocaine, a short acting anaesthetic, has marked vasodilator effects, which limit the duration of action to only 5-10 minutes. It therefore doesn’t have many clinical applications in veterinary dentistry. The addition of adrenaline produces vasoconstriction and a decreased blood flow leading to less bleeding in the area. The decreased blood perfusion leads to slower absorption into the cardiovascular system and more drug remains at the site and therefore an increased duration, extending to 30 minutes.
Mepivacaine, a medium acting anaesthetic, has a rapid onset of action 1.5 minutes, and a longer duration than lignocaine. It can be used quite successfully as the plain drug without vasoconstrictor added.
Chemical formula for mepivacaine.
Prilocaine without added vasoconstrictor is able to provide anaesthesia.
Bupivacaine is available as a 0.5% solution combined with adrenaline. It has a long onset of action, the veterinarian must plan 10 minutes ahead but this is not difficult. It has a long duration for soft tissue anaesthesia. For short surgical procedures, bupivacaine can be administered for pain control. For longer surgical procedures, or when long duration post-operative pain control is required, a shorter acting anaesthetic can be given pre-emptively, and then bupivacaine administered at the end of the procedure, just prior to the patient waking up form general anaesthetic.
Chemical formula for bupivacaine.
The use of vasoconstrictors should be chosen with care. Consider inadvertent use of excessive volumes of local anaesthetic may result in vasoconstriction of the local area with resultant ischemia. Although uncommon, this should be considered when placing local anaesthetics in soft tissues.
Techniques of regional anaesthesia
The anatomy of the head, neck and oral cavity should be reviewed.
The right and left trigeminal nerve provides, amongst other functions, the overwhelming majority of the sensory innervation to the teeth, bone and soft tissues of the oral cavity. The trigeminal nerve is the largest cranial nerve, and comprises a small motor root that supplies the muscles of mastication and other muscles in the area, and a larger sensory root.
The motor fibres supply the following muscles:
- Masseter
- Temporalis
- Pterygoideus medialis
- Pterygoideus lateralis
- Mylohyoid
- Anterior belly of digastric
The sensory fibres are divided into three branches:
- Ophthalmic division
- Maxillary division
- Mandibular division
Ophthalmic division
The ophthalmic division is exclusively sensory and supplies the eyeball, conjunctiva, lacrimal gland, mucous membrane of the nose and paranasal sinuses, and the skin of the forehead, eyelids and nose.
Maxillary division
The maxillary division crosses the pterygopalatine fossa, gives off branches to the posterior superior alveolar nerve (supplies the maxillary molar teeth) and pterygopalatine nerves (supplies the palatal soft tissues), angles laterally towards the infra-orbital canal becoming the infra-orbital nerve and enters the infra-orbital canal. The palatine branches emerge on the hard palate through the greater palatine foramen, located midway between the midline of the palate and the teeth at the level of the anterior border of the maxillary fourth premolar tooth. The nerve courses anteriorly between the mucoperiosteum and the osseous hard palate in a groove, supplying the sensory innervation to the palatal soft tissues and bone as far anteriorly as the canine tooth, where it communicates with the terminal fibres of the nasopalatine nerve.
The posterior superior alveolar nerve leaves the main trunk of the maxillary division just before it enters the infra-orbital canal. It enters the maxilla with a branch of the internal maxillary artery to provide sensory innervation to the alveoli, periodontal ligaments, and pulpal tissues of the maxillary first and second molar and the fourth premolar teeth in the dog; and the molar and fourth pre-molar teeth in the cat.
Within the infra-orbital canal, the maxillary division gives off two branches, the middle superior and the anterior superior alveolar nerves. The middle superior alveolar nerve supplies sensory innervation to the first, second and third premolar teeth in the dog; and the second and third premolar teeth in the cat. The anterior superior nerve provides pulpal innervation to the incisor and canine teeth in both the cat and dog, and sensory innervation to the periodontal ligament, buccal bone and mucous membranes. The infra-orbital nerve emerges through the infra-orbital foramen onto the face to supply sensory innervation to nose, skin and mucous membranes of the upper lip.
Mandibular division
The mandibular division is the largest division and is composed of a large sensory root and a small motor root (see list of muscles previously described). The mandibular division separates into an anterior and posterior branch. The anterior branch is primarily motor and has sensory innervation to the mucous membranes of the cheek and buccal mucous membranes of the mandibular molars. The posterior branch descends parallel to the vertical ramus of the mandible as the inferior alveolar nerve, where it enters the mandibular canal on the lingual surface of the mandible at the level of the mandibular foramen. Throughout its course it travels with the inferior alveolar artery and vein and travels anteriorly in the mandibular canal as far forward as the mental foramen, where it divides into terminal branches, the incisive nerve and mental nerve. The inferior alveolar nerve supplies the mandibular teeth through their apices and provides pulpal innervation. Other fibres supply sensory innervation to the buccal periodontal tissues of the same teeth. The mental nerve exits through the mental foramen and supplies the skin of the lip.
Techniques for local anaesthesia
The techniques for regional anaesthesia of the maxillary teeth require the infra-orbital nerve block, the caudal maxillary nerve block and the greater palatine nerve block. The technique for regional anaesthesia for the mandibular teeth requires the inferior alveolar nerve block and mental nerve block.
Infra-orbital nerve block.
The infra-orbital nerve block in veterinary dentistry works well to block the maxillary teeth and the buccal soft tissues associated with them. The nerve anaesthetised is the infra-orbital and anterior superior alveolar nerves. Indications for this nerve block would include treatment of the maxillary teeth, treatment of the soft tissues of the anterior portion of the mouth. The most common reason for failure to achieve adequate anaesthesia may occur when the tip of needle is not in the correct position. Complications may include a haematoma but this is rare, as it is a relatively simple technique with easy to find landmarks.
Technique
A 30 gauge short needle is recommended. This is an intra-oral injection. The lip should be lifted to reveal the teeth and buccal mucosa. Before injecting make sure: depth of needle is adequate to reach foramen, any deviation of the needle away from the foramen, orientation of the bevel. The bone is palpated approximately 0.5mm in cats and 1-2cm in dogs dorsal to the maxillary third premolar tooth. An outward bulge can be palpated which is the buccal border of the infra-orbital foramen. The bone caudal to this bulge is the border of the infra-orbital canal. The palpable hollow, cranial to the bulge, is the foramen of the canal and the point where you wish to deposit the local anaesthetic. Maintain your finger on the edge of the bulge, applying tension to the tissues. The bevel of the needle is orientated towards the bone. Insert the needle through the mucosa just dorsal to the muco-gingival line at the level of the second premolar tooth in a caudal direction towards the infra-orbital foramen. Advance the needle slowly until bone is gently contacted. The point of contact should be the upper rim of the infra-orbital foramen. The general depth of penetration is to the level of the distal root of the maxillary 4th premolar tooth. Aspirate. Slowly deposit 0.1mls (cat) and up to 0.5mls (dog) over 30 seconds. Maximum volume is 1 cartridge per 10 kg body weight total volume from all sites. Little or no swelling should be visible. You should be able to feel the solution under your finger. Move your finger over the exit of the foramen and slowly withdraw the needle. Maintain direct finger pressure for another 30 seconds.

Infra-orbital nerve exiting the rostral foramen of the infra-orbital canal (blue line) in a dog.
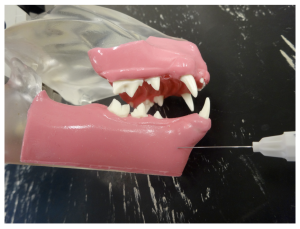
Infra-orbital nerve block in a dog. Insertion of the needle through the buccal mucosa towards the rostral foramen of the intra-orbital canal.
Infra-orbital nerve exiting the rostral foramen of the infra-orbital canal (blue line) in a cat.
Infra-orbital nerve block in a cat. Insertion of the needle through the buccal mucosa towards the rostral foramen of the intra-orbital canal.
Caudal Maxillary nerve block.
The caudal maxillary nerve block in veterinary dentistry works well to block the maxillary teeth and the buccal soft tissues associated with them. The nerve anaesthetised is the maxillary, infra-orbital and anterior superior alveolar nerves. Indications for this nerve block would include treatment of the maxillary teeth, treatment of the soft tissues of the anterior portion of the mouth. The most common reason for failure to achieve adequate anaesthesia may occur when the tip of needle is not in the correct position. Complications may include a haematoma, perforation of the eye and neuritis.
Technique
A 30 gauge long needle is recommended. This is an intra-oral injection. The mouth should be opened fully to reveal the teeth and caudal soft palate. The caudal edge of bone on the hard palate is palpated disto-palatal to the molar teeth. The palpable hollow, distal to the bone edge is the ventral surface od the pterygo-palatine fossa in which the maxillary nerve lies and the point where you wish to deposit the local anaesthetic. Insert the needle through the mucosa just distal to the hard palate into the fossa. Advance the needle slowly level to a distance level with the ventral border of the zygomatic arch. Aspirate. Slowly deposit 0.1mls (cat) and up to 0.5mls (dog) over 30 seconds. Maximum volume is 1 cartridge per 10 kg body weight total volume from all sites. Little or no swelling should be visible. You cannot feel the solution under your finger.

Placement of the needle through the mucosa disto-palatal to the last molar tooth (110) in a dog.

Placement of the needle through the mucosa disto-palatal to the last molar tooth (109) in a cat.
Greater palatine nerve block
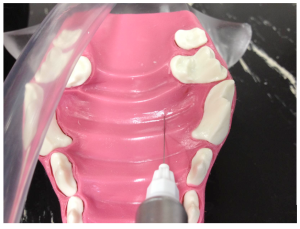
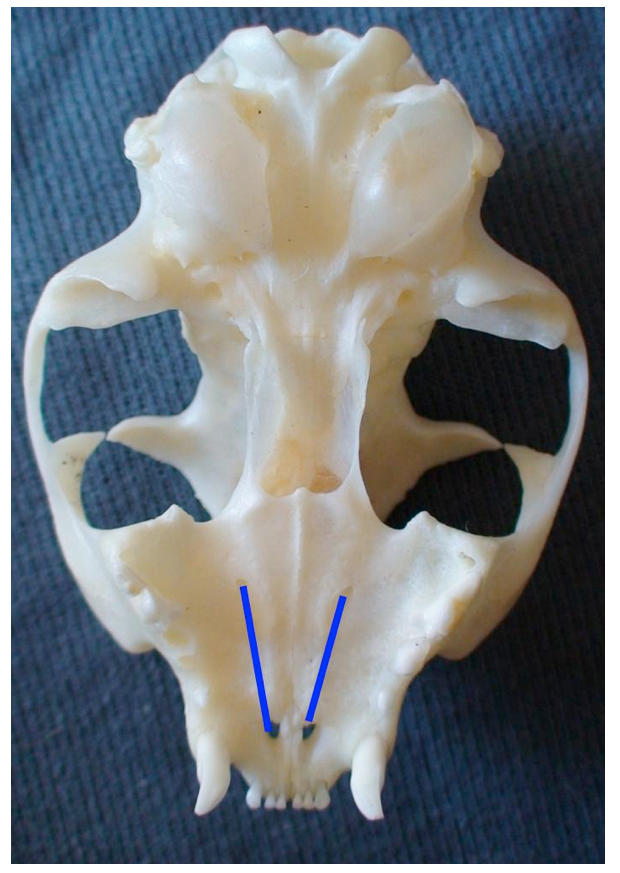
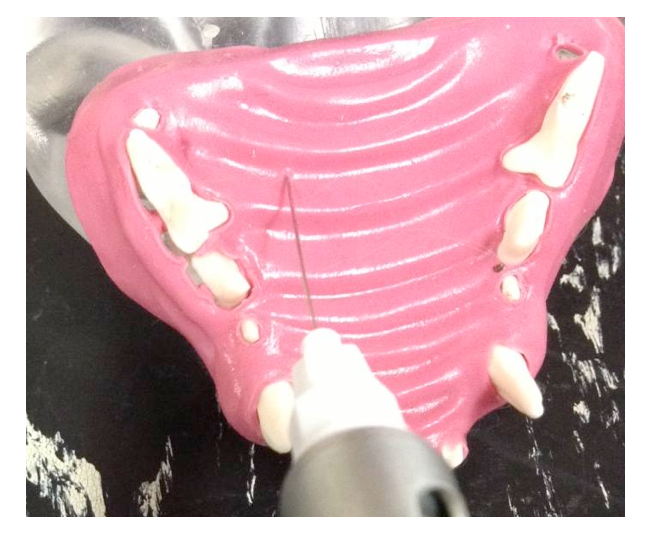
The greater palatine nerve block is used when manipulation of the palatal soft tissues are manipulated distal to the canine. The greater palatine foramen is located midway between the palatal midline and the palatal border of the anterior border of the maxillary fourth premolar tooth. A 30 gauge short needle is recommended. The needle is inserted, with the bevel towards the bone, through the mucosa slightly anterior to the greater palatine foramen. Advance the needle slowly until bone is gently contacted. The general depth of penetration is 2mm in the cat and 5mm in the dog. Aspirate. Slowly deposit 0.1mls (cat) and 0.25mls (dogs) over 30 seconds. Maximum volume is 1 cartridge per 10 kg body weight total volume in all sites. You should be able to see the solution under the mucosa. Slowly withdraw the needle.
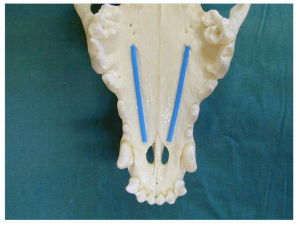
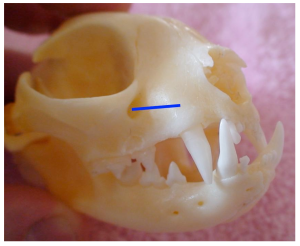
Hard palate with positions of the palatine nerve in a dog (blue line).
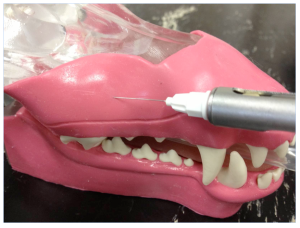
Greater palatine nerve block in a dog. Insertion of the needle through the hard palate mucosa towards the palatine foramen.
Hard palate with positions of the palatine nerve in a cat (blue line).
Greater palatine nerve block in a cat. Insertion of the needle through the hard palate mucosa towards the palatine foramen.
Mandibular anaesthesia
Achieving clinical analgesia in the maxillary is much easier than in the mandible. The bone is less dense in the maxilla and there is easy access to the large nerve trunks compared to the anatomical variations and the thickness of the bone in the mandible. The nerve block in the mandible involves blocking the inferior alveolar (mandibular) nerve as it enters the mandible on the lingual surface at the posterior area of the mandible. The nerve from this point supplies all teeth and soft tissues.
Inferior alveolar nerve block.
The inferior alveolar nerve block anaesthetises the inferior alveolar branches of the posterior division of the mandibular nerve, the incisive nerve, and the mental nerve. It is used for procedures on multiple teeth in one mandibular quadrant, when buccal soft tissue anaesthesia or lingual soft tissue anaesthesia is required. Contra-indications are when infection is present in the area of the injection and in patients that may bite or self-mutilate the tongue after anaesthesia.
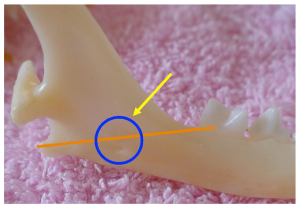
An intra-oral approach is used to locate the mandibular foramen on the lingual surface of the mandible at a point half way along an imaginary line drawn between the last molar tooth and the angular process of the mandible. In large patients the mandibular foramen can be palpated by placing a finger intra-orally and palpating the lingual surface of the mandible at caudal to the last molar tooth. In smaller patients or when the foramen cannot be palpated, the position of the foramen can be determined at a point midway between the last molar and angular process. This is achieved by placing the veterinarian’s finger and thumb on the outside of the the patient’s head and the thumb intra-orally. The thumb is placed on the last molar tooth intra-orally and the forefinger on the angular process extra-orally, the mandibular foramen can be estimated to be halfway between these points.
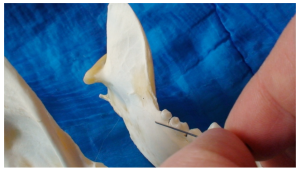
A 30 gauge short needle (cat) and long needle (dog) is recommended. The mouth should be opened wide and the lip should be pulled laterally and the tongue reflected to the opposite side of the mouth. The bevel of the needle is orientated towards the bone. Insert the needle through the mucosa caudal to the last molar tooth towards the angular process. Advance the needle slowly until bone is gently contacted. The needle is advanced along the bone towards the entrance of the foramen until the tip is at the halfway point. The general depth of penetration is 10mm in the cat, 15mm in a small dog and 20mm in a large dog. Before injecting make sure: depth of needle is adequate. Do not deposit local anaesthetic if bone is not contacted. Aspirate. Slowly deposit 0.1mls (cat) and up to 0.5 mls (dog) over 30 seconds. Maximum volume is 1 cartridge per 10 kg body weight total volume in all sites. Slowly withdraw the needle. Wait for the anaesthetic to work.
\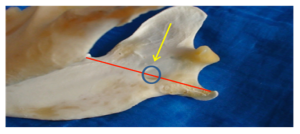
Diagram showing position of mandibular foramen in a dog (the red line represents the distance from the mandibular 3rd molar to the angular process, the circle is the mandibular foramen highlighted by the yellow arrow.
Desired direction of intra-oral mandibular block in a dog.
Desired direction of intra-oral mandibular block in a dog.
Diagram showing position of mandibular foramen in a cat (the red line represents the distance from the mandibular 3rd molar to the angular process, the circle is the mandibular foramen highlighted by the yellow arrow).
Desired direction of intra-oral mandibular block in a cat.
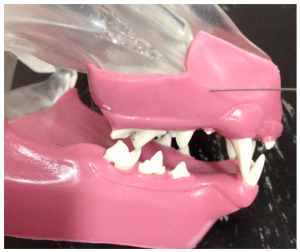
Mental nerve block
The mental nerve block anaesthetises the inferior alveolar branches of the mandibular nerve rostral to the 2nd premolar tooth. It is used for procedures on canine and incisor teeth.
An intra-oral approach is used to locate the mental foramen on the buccal surface of the mandible caudal to the lip frenulum ventral to the 2nd premolar tooth. A 30 gauge short needle is recommended. The upper lip is raised and the lower lip pulled ventrally to expose the frenulum. The bevel of the needle is orientated towards the bone. Insert the needle through the mucosa cranial to the frenulum towards the foramen. Advance the needle slowly until it enters the foramem. The general depth of penetration is 2-3mm in the cat, 5mm in a dog. Aspirate. Slowly deposit 0.1mls (cat) and 0.5mls (dog) over 30 seconds. Maximum volume is 1 cartridge per 10 kg body weight total volume in all sites. Slowly withdraw the needle. Wait for the anaesthetic to work.
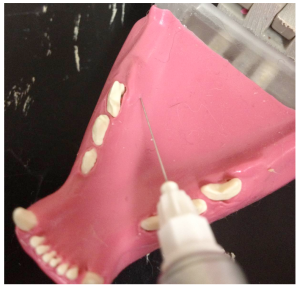
Mandible of a dog demonstrating position of mental formamen (distal mental formamen [left] and middle mentalforamen [right].
Middle mental nerve block in a dog. Insertion of the needle through the mucosa towards the middle mental foramen.
Mandible of a cat demonstrating position of the middle mental formamen.
Middle mental nerve block in a cat. Insertion of the needle through the mucosa towards the middle mental foramen.
Conclusion
With the rise in interest in small animal welfare and the concern with pain prevention by both the dog and cat owner and the companion animal veterinarian, analgesia during dental procedures should be a serious consideration. Local anaesthetic nerve blocks have enabled human dentistry to advance in leaps and bounds. As they have now been proven to work clinically in veterinary dentistry, analgesia should be available to all small animal patients.
References
- Barnett JL (1997) Measuring pain in animals. Aust Vet J 75:878
- Carroll G. (1999) Analgesics and pain. Vet Clin Nth Am Small Animal Pract 29:701
- Clarke DE. (2004) The physiological effects of peri-operative analgesia in dogs undergoing tooth removal.
- Aust Vet Practit 34:62
- Covino BG and Vassallo HG. (1976) Local anaesthetics: mechanisms of action and clinical use, New York, Grune and Stratton de Jong RH and Wagman IH. (1963) Physiological mechanisms of peripheral nerve block by local anesthetics. Anesthesiology 24:684-727
- Fitzgerald MJT (1992) Neuroanatomy: basic and clinical, London, Bailliere Tyndall
- Gaynor J (1999) Is postoperative pain management important in dogs and cats? Vet Med 94:254
- Hendrix P and Hansen B (2000) Kirk’s Current Veterinary Therapy Small Animal Practice, Ed Bonagura, p57, Saunders, Philadelphia
- Iwatsubo T, Hirota N and Ooie T (1997) Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol Ther, 73:247-271
- Lascelles BDX, Waterman AE, Cripps PJ, Livingston A and Henderson G (1995) Central sensitisation as a result of surgical pain: investigation of the pre-emptive value of pethidine of ovariohysterectomy in the rat, Pain 62:210
- Malamed SF (2004) Handbook of local anesthesia, ed 5, Mosby, St Louis
- Strichartz GR and Ritchie JM (1987) The action of local anaesthetics on ion channels of excitable tissues. In Local anaesthetics Strichartz GR, editor, New York, Springer-Verlag
- Wall PD (1988) The prevention of post-operative pain. Pain 33:289
- Woolf CJ (1989) Recent advances in the pathophysiology of acute pain Br J Anaes 63:139
- Woolf CJ and Wall PD (1986) Relative effectiveness of C primary afferent fibres of different origins in evoking a prolonged facilitation of the flexor relex in the rat. Neurosci 6:1433


![Mandible of a dog demonstrating position of mental formamen (distal mental formamen [left] and middle mentalforamen [right]](/wp-content/uploads/2021/11/Mandible-of-a-dog-demonstrating-position-of-mental-formamen-distal-mental-formamen-left-and-middle-mentalforamen-right.png)